A closer look at tears leads to potential strategies for treating vision loss

When Brian McKay, PhD, graduated from college with a biology degree in the 1980s, it was a time of transition. He got married that same week and left behind his restaurant job to launch a search for a permanent career.

“I wanted to do something with my degree,” he recalled. “There was one job opening in science, and it was in an eye lab.”
His career trajectory was defined in that lab. After a couple of years, he returned to school to delve deeper into the eye’s mysteries. Now a professor of ophthalmology and vision science at the University of Arizona College of Medicine – Tucson, he is hard at work cementing his legacy. His discoveries in how the eye works — and fails — are leading to better ways to detect and treat vision loss.
He is particularly interested in age-related macular degeneration (AMD), which as of 2019 causes vision impairment or blindness in an estimated 1.49 million Americans. People with advanced AMD have “blurry” or “wavy” central vision, causing difficulties with formerly simple tasks — recognizing faces, driving a car, reading a book.
“When you meet them, you know why it’s such a terrible disease,” Dr. McKay said. “They lose their ability to connect with their family, to see their grandchildren. They never see them smile. They have to look to the edge of their visual field.”
Tiny molecules trigger huge vision problems
The McKay Lab focuses on an area of the retina called the retinal pigment epithelium (RPE), which maintains light-capturing cells called photoreceptors. As we age, RPE cells slowly die out and are unable to do this maintenance work. The early stages of AMD don’t cause vision impairment, but as we age, risk of vision impairment or blindness caused by late-stage AMD climbs. Fewer than half a percent of people in their late 60s suffers from late-stage AMD, but 18% of people living into their late 90s reach this stage.

“When RPE cells fail to reconstruct broken photoreceptors, the photoreceptors break down,” Dr. McKay said. “That’s probably where blindness comes from.”
In late-stage AMD, blood vessels invade the back of the eye, leaking fluid into the macula — the part of the retina that controls straight-ahead vision. These unwelcome blood vessels are thought to be summoned by exosomes, microscopic packets of fluid excreted by RPE cells. The more exosomes that are produced, the louder that signal becomes, speeding up abnormal blood vessel formation and accelerating macular degeneration.
The McKay Lab was one of the first to investigate these minuscule molecules in the eye, which have been a research focus for nearly two decades. Exosomes are thought to help cells communicate with one another, and tracking their numbers over time might give doctors a glimpse at a patient’s vision health.
“We’re the only lab in the state that can purify exosomes, see them, count them and deal with them. We have the equipment and we have the expertise,” he said.
Harvesting tears to track health
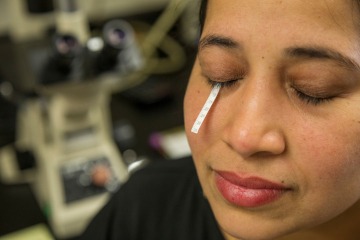
The McKay Lab developed a process for collecting and analyzing exosomes using a patented “tear strip,” a small piece of absorbent gauze inserted into the lower eyelid to collect a patient’s tears — which include measurable levels of exosomes.
“I take this little strip, I put it in the eye, and five minutes later, I pull it out,” he said. “The exosomes are then harvested, and we count them in the lab.”
Since RPE cells start excreting abnormal levels of exosomes well before symptoms start, the tear strips could be used to monitor people with early-stage AMD, as well as those at risk of other vision problems caused by excess blood vessels, such as diabetic retinopathy and glaucoma. When exosome production reaches a certain threshold, it might be time to start preventive treatment. At that point, exosomes may still be monitored to track the treatment’s effectiveness.

“We don’t really have a good way of knowing whether these things are working until they fail. The only way we know they failed is the person went blind — not helpful!” Dr. McKay said. “If I can do a five-minute noninvasive biopsy for these different diseases and see whether the treatment is working, I could follow this over time.”
The McKay Lab is working with clinical partners to learn more about how exosome levels correspond to disease progression, and if they can be used to figure out when to start treatment and to monitor its effectiveness. If they are successful, the hope is that patients at risk for vision loss could someday have realistic solutions for nipping blindness in the bud.
A new use for an old drug
Exosomes are just one focus of the McKay Lab. They are also investigating pharmaceuticals for AMD, which is currently treated with an injection — directly into the eyeball — of a drug that shuts down abnormal blood vessel formation. Dr. McKay hopes to find an even better treatment in the form of an inexpensive medication that’s been used widely for decades: L-DOPA.

It all started when Dr. McKay was looking for ways to “talk” to RPE cells through cell receptors — tiny proteins that allow cells to communicate with one other through chemical signals. If a drug could attach to a receptor, it might be able to order the cell to maintain photoreceptors and shut down abnormal blood vessel formation. Eventually, Dr. McKay discovered that RPE cells are studded with receptors that he dubbed GPR143 — and that L-DOPA binds to them.
Curious, he wondered if people with Parkinson’s disease — who take L-DOPA to control their symptoms — are at lower risk for AMD. Looking at decades’ worth of data to compare people with Parkinson’s to the general population, he found an inverse correlation between Parkinson’s and AMD.
“That was a study of a quarter of the population of the United States, 84 million people,” he said. “People taking L-DOPA for Parkinson’s are much less likely to get AMD, and if they do, they get it about eight years later.”
What Dr. McKay learned about this receptor, combined with the lower prevalence of AMD among people with Parkinson’s, provided justification for a clinical trial investigating L-DOPA in people with AMD. He hopes that, by activating the receptor, L-DOPA could induce RPE cells to stop sending exosomes calling for new blood vessels and to continue maintaining photoreceptor cells.
“The first clinical trials were done here in Tucson,” he said. “We gave them L-DOPA, and they saw better in four days. They had a 53% reduction in the necessity of getting injections again.”
Dr. McKay says that L-DOPA appears to shut down blood vessel formation by decreasing exosome release and turning off an additional molecule that helps blood vessels grow, while also triggering production of a molecule that protects photoreceptors.
“Our receptor turns off the bad stuff and turns on the one thing that’s good,” he said. “It’s a great receptor!”
Looking ahead to a bright future

Dr. McKay has been overseeing his lab since 1997, first at Duke University before coming to the College of Medicine – Tucson in 2002. He hopes that, by the time he’s ready to retire, he’ll have found a way to detect vision loss early by monitoring exosomes, and that he’ll expand the treatment options available by adding L-DOPA to the toolkit. If successful, he believes his lab will have accomplished something monumental.
“There’s a large community of aging people who would really like to have something actually work for this disease,” he said. “I hope I can tell them, ‘Here’s the cure. It took me a while, and a lot of work, but I did it!’ It’s one thing, but it’s a big thing.”
Looking back, Dr. McKay doesn’t regret the paucity in job openings after receiving his bachelor’s degree, because the position in the eye lab got him exactly where he needed to be.
“I got the job, and I’ve been an eye person since,” he said. “I either became an eye person, or I always was one and didn’t know it!”

