About Us
The Graduate Medical Education (GME) Research Committee at the University of Arizona College of Medicine – Tucson is dedicated to fostering a vibrant research environment for medical trainees at all levels. The committee’s mission is to provide comprehensive resources and facilitate networking opportunities, empowering trainees to engage in original research projects. By offering access to research-related resources, mentorship and funding opportunities, the GME Research Committee aims to cultivate a supportive and collaborative atmosphere. This enables trainees to explore innovative ideas, develop critical research skills and contribute meaningful findings to the medical field, ultimately enhancing their academic and professional growth.
Research 101
There is a lot of clinical research-related education available online. The following primer was written by Dr. Spencer Moore, ophthalmology resident and co-director of education for the GME Research Committee.
Chronicity
Retrospective: the entire event being studied started, took place, and was completed in the past
Cross-sectional: only a single point in time
Prospective: recruit subjects in present and follow longitudinally over time
Groups
Single-armed: only 1 group (e.g., people who smoke)
Double-armed: multiple groups studied (e.g., control/placebo arm and intervention/exposure arm)
Intervention
None/observational: subjects are studied for the association between an independent variable (e.g., smoking status) and dependent variable/outcome (e.g., development of lung cancer)
Interventional: subjects are grouped according to some treatment or intervention received
Masking
Single-masked: subjects are masked to the status of treatment vs. placebo
Double-masked: subjects are masked to the status of treatment vs. placebo AND investigator is masked to subjects’ group assignment
Randomization
Only possible for prospective interventional cohort studies; avoids selection and allocation biases inherent to nonrandomized study design. Allows for evidence of causality between independent (e.g., treatment/intervention) and dependent (e.g., outcome) variables.
A well-referenced and concise written research proposal is essential. Depending on where you are sending it (UA RIA, UA IRB, SAVAHCS, Banner, private funding agencies, etc.), there may be differences in the format. In general, the format will include the following elements:
Abstract
May or may not be included depending on format. It should be 1 paragraph and about 250-300 words long. It would include a brief few sentences of background and introduction. This should lead into the knowledge gap in the field and set up the reader for the hypothesis to be tested. Next, the study design and aims should be stated as briefly and concisely as possible. Next, the hypothesis should be stated, followed by the importance or significance of potential results in the field. The abstract generally does not contain references.
Background & Introduction
Should be about 1 page or less of single-spaced background and introduction to the topic being studied, usually for a general medical or scientific audience. This section should be well referenced. Introduce the condition being studied and some of its public health implications. Then describe what is known in the field about your specific topic. This should progress logically toward the question your study seeks to answer. You may conclude this section with something to the effect of, “In the present study, we plan to use [insert study design here] to answer the question of [insert major research question or knowledge gap here].”
Specific Aims
Depending on the format of the study, this may be a single aim with several sub-aims, or multiple specific aims, each with their own sub-aim. Describe the format of the study, the patient population to be studied, means of recruitment, data points to be collected, type of statistical analysis and comparisons to be performed. Include a testable hypothesis related to the aim described. Each aim should be about 1 paragraph.
Protocol
This will include a detailed account of the experimental protocol. It may be several pages long. It will vary according to your specific project. Organize it according to each specific aim. It will include a detailed description of the patient population being studied, how those patients will be selected or recruited, the source of data to be collected, the data points that you will collect and how those data will be analyzed. You should also include a selection relating to human subjects protection that describes how clinical data will be safeguarded, how human subject identities will be protected and deidentified, how data will be stored, etc. If subjects are being actively recruited and consented for a study there will be an additional section describing the informed consent process and how subjects will be recruited. Include a primary outcome measure in your data to be collected and a description of how this will be calculated. For example a primary outcome measure in a prospective observational cohort study of smokers and nonsmokers might be the development of lung cancer within 10 years, and the statistical analysis to be performed might be relative risk of developing lung cancer within 10 years of enrollment. If there is any prospective component to your research component, where patients are brought in for testing or treatment, then you will need to identify a funding source — your research mentor can assist you.
Power Analysis
For a prospective study, it is important to have some statistical basis to calculate the number of subjects you plan to recruit. For a retrospective study, this can also be helpful to determine the number of charts needed to review. There are various online sample size calculators as part of your power analysis.
It is important to have a reasonable grasp of statistics in your research protocol. Understand exactly what clinical data will be collected so that you can propose the appropriate statistical analysis.
Some common statistical tests:
t test: to compare means of 2 groups with continuous numerical data. Generally, it would be a 2-tailed, unpaired t-test. For example, you could compare the mean age of 2 groups.
Chi-square or Fisher exact test: compares the proportions of 2 subjects in 2 groups. For example, you could compare the percentage of subjects in 2 groups who develop lung cancer.
ANOVA: to compare group means of a dependent variable with multiple different levels of the independent variable, for example systolic blood pressure levels with different dosages of blood pressure medications.
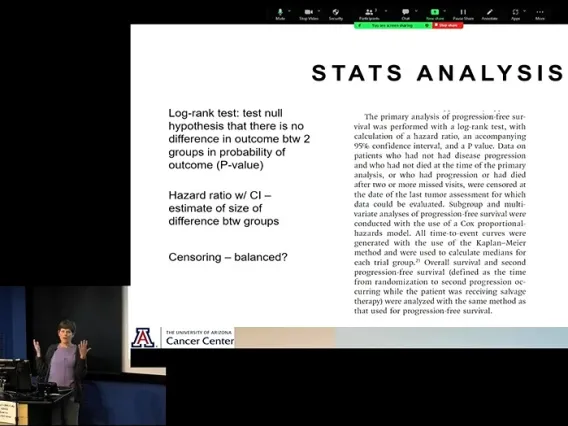
Kaplan Meier: comparing survival or latency to meet some primary outcome measure over time. Can calculate a Kaplan Meier or log-rank statistic to compare survival in 2 different groups.
Tips
You will probably need 2 separate and parallel research applications approved before starting your project if using the UA research infrastructure and IRB. Your department likely has research coordinators who can help you and determine any documents you will need. This will probably include approvals from the Research Intake Application (RIA) and an Institutional Review Board (IRB).
UA REDCap is a great secure way to store clinical data. If you will be collecting lots of separate data points, REDCap is an efficient way to store, organize and sort data that can easily be anonymized and exported.
Step by Step: Conducting Research
Conducting research as a trainee can be daunting. Consider the following steps to get you started with conducting a human research project, and please also discuss with your mentor. For an explanation of the full IRB process, review the “Getting Started” webpage at RII.
Complete CITI Human Subjects Protection training
Complete the Biomedical Research BASIC Course at CITI Program.
Download IRB forms
Download the appropriate forms from the IRB website:
- IRB protocol
- consent forms
- applicable appendices
- attestations from your adviser, scientific reviewer, and department head
Begin eIRB submission
Begin your submission at the eIRB website (NetID required). This how-to video may help you to complete this step.
If applicable, submit to Banner
If Banner staff, patients, medical records or specimens will be used, submit your study through the Research Administration Portal (RAP) (NetID required).
Add Banner approval to eIRB
Once RAP is approved, add the approval email to your study in eIRB and press “Submit” for your IRB to be formally submitted. An IRB coordinator will be assigned to pre-review your study prior to final IRB chair review/approval. Anticipate receiving comments and questions for further revision during this phase. Once the study is approved, you will receive an eIRB system-generated email. Make sure to review your approval letter to understand reporting requirements moving forward. You can then begin your research.
Work with statistician
Once the data from the project is available for analysis, work with a statistician to help with the pre-defined statistical analysis plan, and interpret the results to draw meaningful conclusions related to the research question.
Share your results
Consider presenting your research at local and or national meetings, as you are collecting your data with the ultimate goal of a peer-reviewed manuscript publication.
Additional Resources
Please type keywords into UA Profiles to identify a mentor with similar research interests.
Details to follow for GME Scholarship opportunities.
GME Research Chair Salma Patel, MD, MPH, holds office hours on most Mondays from 2-3 p.m.
Feel free to drop in on Zoom or send her an email to set up a different time to meet.


![[Salma Patel, MD, MPH, FACP, FAASM]](/sites/default/files/styles/az_medium/public/2025-09/Patel-Salma-MD-MPH_PACCS_Headshot_8x10.jpg.webp?itok=fO16M6w-)