Neurosurgeon’s journey home takes clinical research to the next level
Dr. Paul Larson envisions a future for functional neurosurgery that relies on precise delivery of cell-altering genes and lab-grown brain cells.

As part of the MRI-guided surgical technique they pioneered for treating Parkinson’s disease, Dr. Paul Larson and his collaborators developed a device that temporarily attaches to the skull to help pinpoint a precise location in the brain.
Kris Hanning, University of Arizona Health Sciences
Professor of Neurosurgery Paul S. Larson, MD, FAANS, was shaped in Arizona, from his childhood in the East Valley of Phoenix and his undergraduate years in Tempe, culminating with his medical education at the University of Arizona College of Medicine – Tucson. He then spent two decades at the University of California, San Francisco, where he pioneered new techniques in brain surgery, but Arizona beckoned him home.
Now he is back at the College of Medicine – Tucson, and has brought his flair for innovation along. He recently completed a first-of-its-kind procedure to treat Parkinson’s disease that involved transplanting healthy cells into his patient’s brain.
“Parkinson’s is caused by a loss of a kind of brain cell that controls our movements,” he said. “When you lose those cells, you get abnormal activity causing tremor, stiffness of movements, slowness.”
These dying cells once made dopamine, a chemical messenger brain cells use to communicate among themselves and with the rest of the body. Some of the earliest signs of Parkinson’s involve movement symptoms — shaking hands, trouble walking, changes in posture and even handwriting.
“Symptoms are usually mild at first and well controlled with medications. As the years go by, the meds become less effective,” Dr. Larson said. “Alzheimer’s is the No. 1 most common neurodegenerative disorder. Parkinson’s is No. 2. About 1% of people over the age of 50 have it.”
‘I got hooked’
He always wanted to be a surgeon, though it wasn’t until his senior year at Arizona State University that Dr. Larson set his sights on the brain.
“I took a neuroscience course to fulfill a requirement to graduate. It was riveting,” he recalled. “Of all of the parts of the body, we understand the brain the least — but it makes us who we are. I got hooked.”
By the time he started medical school, he was laser focused on his future career.
“I came knowing I wanted to study the brain,” he recalled.
“The brain makes us who we are. I got hooked.”
After receiving his medical degree, Dr. Larson followed his interest to a subspecialty called functional neurosurgery, which acts directly on the nervous system to treat pain and movement disorders like Parkinson’s disease and epilepsy. He takes advantage of the electrical signals that naturally pulse across the brain as cells communicate. As Parkinson’s symptoms worsen, this “conversation” is increasingly jumbled. A small, battery-powered device implanted in the chest and connected to electrodes threaded into the brain produces mild electrical pulses that moderate a more orderly conversation between brain cells.
“It’s essentially a pacemaker that is put into the brain. By sending the proper signal, you can make symptoms better,” he said. “It’s become standard of care for Parkinson’s patients when medications aren’t working like they were initially.”
‘You lay the groundwork for others’
Dr. Larson says this technique, called deep brain stimulation (DBS), can help control movement symptoms for a decade or more. It had been developed in the 1990s and approved by the FDA in 2002. Excited by this new surgical strategy, Dr. Larson and his partners at UCSF — Philip Starr and Alastair Martin — started to explore the possibilities. Rather than scanning the brain before the procedure to find a spot to insert the electrode, they used concurrent imaging to place it.
“To take out brain tumors, to make sure they got it all out, people were starting to do brain surgeries inside MRI scanners, which allow you to see inside the brain,” he recalled. “The three of us started thinking, MRI is good for taking things out of the brain — maybe it’s good for putting things into the brain. We developed a way of using real-time MRI while inserting the electrode to make sure it’s going to the right place. It has evolved into this widely used technique — image-guided DBS.”
Like most scientific discoveries, the technique enabling image-guided DBS has applications beyond Parkinson’s, and has since been used to treat epilepsy and deliver targeted drugs directly to the brain.
“What’s good for one problem can be translated across different diseases,” Dr. Larson said. “Even if you don’t make the big discovery yourself — most of us won’t — you lay the groundwork for others to come after you. The cool thing about this field is there are really opportunities for discovery.”
‘The next wave’
As exciting as it is to use mild electrical pulses to correct the brain’s misfires, these techniques don’t prevent or reverse damage to brain cells. Dr. Larson is also interested in surgical procedures that can improve the cells themselves.
“Applying electrical stimulation to the brain doesn’t change the underlying biology,” he said. “Biologic approaches to these diseases is the next wave — things like gene therapy and cell transplantation. DBS can help with the symptoms you already have, and biologics can slow the progression of disease. A combination approach may be what things look like 10 years from now.”
These days, Dr. Larson is conducting gene therapy research in Huntington’s disease, another neurodegenerative condition, and is collaborating with groups using the same techniques in Parkinson’s. The hope is that, by correcting a malfunctioning gene, doctors can help the brain cells become healthier.
“Genes are like recipes that our cells use. In Parkinson’s, brain cells are dying,” he said. “We’re using viruses as taxi cabs to carry a gene for something called growth factors. We deliver these gene-carrying viruses to the brain. Within hours, you can see effects in the brain cells. If trials are successful, you may be able to get the brain to produce growth factor and slow the progression of Parkinson’s.”
‘A very exciting time for cell transplantation’
In addition to gene therapy, Dr. Larson is pioneering new methods of cell transplantation involving brain cells made from a patient’s own skin cells, a personalized or “autologous” approach.
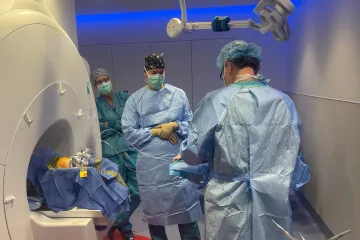
Dr. Larson leads the first brain cell transplant in the Aspen trial using a Parkinson’s patient’s own cells.
Photo courtesy Aspen Neuroscience
“We can take a skin biopsy. It’s no different than going to the dermatologist, which people are very used to doing in Arizona,” Dr. Larson said. “Our research partners biologically reprogram a type of skin cell into tens of millions of dopamine-producing cells, and we then put the patient’s own cells into their brain. The immune system shouldn’t attack them.”
Today, he combines these advances in cell transplantation with the technique he pioneered when developing image-guided DBS. Earlier this year, at Banner – University Medicine Tucson, Dr. Larson performed the first autologous cell transplants in Parkinson’s patients in a clinical trial sponsored by Aspen Neuroscience, using MRI to guide him in real-time.
“The hope is these cells will survive and release dopamine,” he said. “We have people really excited to be part of these trials, to help us advance the field and come up with new therapies — not just for them, but for the whole Parkinson’s community. It’s a very exciting time for cell transplantation.”
‘Good to be back’
Dr. Larson wonders if people with Parkinson’s might be more creative on average, pointing to the wall in his office. It is covered with paintings, drawings, handwritten poems and handcrafted textiles — reminders of why he comes to work every day.

Kris Hanning, University of Arizona Health Sciences
“The walls have little knickknacks patients have given me over the years. After DBS, a patient with tremor is back to what they like doing,” he said. “You can make a positive impact in people’s lives — it’s pretty immediate. What we do really helps people. It’s incredibly meaningful to me.”
Dr. Larson was drawn back to the College of Medicine – Tucson by its collaborative culture and innovative spirit.
“It was a very formative time, and set the tone for the rest of my career,” he said. “It’s good to be back where all that started.”

